Abstract
Background: Therapy-related AML (t-AML) is a rare and fatal complication in cancer patients (pts) previously treated with chemotherapy (CTX) and radiotherapy (RT). The risk of t-AML has evolved over time mostly due to advances in cancer therapeutics. In this study, we provide a comprehensive analysis on the risk of t-AML over a 40-year period in cancer patients treated with CTX with or without RT in the US.
Methods: Eligible pts for this study were identified from nine Surveillance, Epidemiology, and End Results Program (SEER 9) registries and included those between the ages of 18 and 84 years who were diagnosed with any first primary malignancy (other than CML) between 1975-2018 and received initial course of treatment with CTX. All AML cases that occurred at least one year after the first diagnosis of a primary malignancy were included as t-AML as per the World Health Organization classification. All malignancies were identified based on International Classification of Disease for Oncology, 3rd edition (ICD-O-3) morphology and topography codes. Subjects were followed until t-AML diagnosis, attained age of 85 years, death, loss to follow up, or end of study (December 31, 2018).
Standardized incidence ratios (SIRs) were calculated as the ratio of the observed-to-expected (O/E) number of AML using SEER*Stat. The expected number of AML was computed from age-, race-, sex-, and calendar year-specific incidence rates of AML from the general SEER population, multiplied by the appropriate person-years at risk. We calculated exact 95% confidence intervals (CI) about the SIRs. We also computed the excess absolute risk (EAR) to estimate the number of excess cancers beyond that expected per 10,000 persons per year. We conducted Poisson regression analyses comparing excess relative risks (ERR) with initial RT, using CTX alone as the reference.
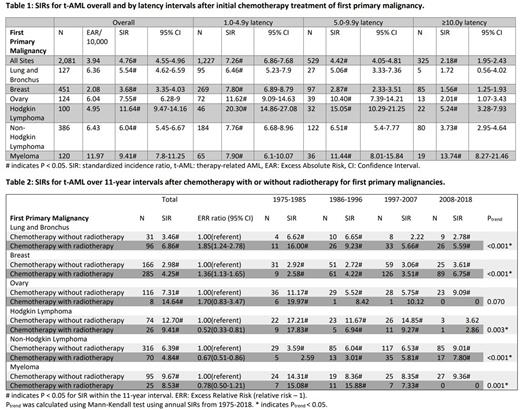
Results: Among 1,030,380 pts initially treated with CTX for first primary malignancy, we identified 2,081 t-AML cases with a 4.76 fold increased risk of AML (SIR; 95% CI, 4.55-4.96) compared to the general population and an excess of 3.94 cases per 10,000 person-years (Table 1). We focused our analysis on first primary malignancies with at least 100 t-AML cases, which included cancers of the lung, breast, ovaries, Hodgkin Lymphoma (HL), Non-Hodgkin Lymphoma (NHL), and myeloma. t-AML risks were particularly high (six- to twenty-fold higher than general population) in the first 5 years following these primary cancer diagnoses, then declined substantially with increasing latency. Long-term risk (≥10 years) of t-AML remained significantly elevated in pts with all these primary cancers except for lung cancer and the risk was highest in pts with myeloma (SIR = 13.74; 95% CI, 8.27-21.46).
Additionally, we assessed the risk of t-AML with and without RT in addition to CTX (Table 2). Pts were more likely to develop t-AML if they were treated with both RT and CTX for lung cancer (ERR 1.85, 95%CI, 1.24-2.78), breast cancer (ERR 1.36, 95%CI, 1.13-1.65), and ovarian cancer (ERR 1.70, 95%CI, 0.83-3.47) compared to pts who were treated with CTX alone. When analyzed by different calendar periods of diagnosis, the risk of t-AML in pts with HL, NHL and myeloma was lower following combined modality compared to chemotherapy alone in recent time periods (1997-2007, 2008-2018) compared to earlier years of diagnosis.
We also identified trends of t-AML risk over time, displayed in 11-year intervals from 1975-2018 (Table 2). Ptrend was calculated using annual SIRs over the study period. In patients who received CTX for their first primary malignancy, irrespective of RT, we found that t-AML risk has significantly declined after CTX for lung cancer (Ptrend < 0.001), HL (Ptrend = 0.003), and myeloma (Ptrend = 0.001). Over the same period, the risk of t-AML increased in breast cancer (Ptrend < 0.001) and NHL (Ptrend < 0.001).
Conclusions: In our study spanning the past 40 years, we observed the risk of t-AML trending down for lung cancer, HL, and myeloma while increasing for breast cancer and NHL. A notable finding was increasing risk of t-AML with increasing latency in myeloma. Of interest, the risk of t-AML with combined modality regimens (CTX and RT) in recent time periods is lower than chemotherapy alone for HL, NHL and myeloma. Based on these observations, it is imperative to analyze the impact of newer therapies on the risk of t-AML, which will greatly benefit risk-benefit counseling of pts at individual level.
Disclosures
Gerds:Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Accurate Pharmaceuticals: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kratos Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding; Imago BioSciences: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Advani:Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunogen: Research Funding; OBI: Research Funding. Carraway:BMS: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Other: DSMB; AbbVie: Other: DSMB; Syndax: Other: DSMB. Mukherjee:AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Celgene/Acceleron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; BioPharm: Consultancy; Aplastic Anemia and MDS International Foundation: Honoraria; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Genentech: Membership on an entity's Board of Directors or advisory committees; McGraw Hill Hematology Oncology Board Review: Honoraria, Other: Advisor or review panel participant; Partnership for Health Analytic Research, LLC: Honoraria; Jazz Pharmaceuticals: Other: Principal investigator for Investigator Initiated Trials (the Institution gets the funding), Research Funding; Eusa Pharma: Consultancy, Other: Advisor or review panel participant; Teaching and Speaking.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal